Numan Operations Limited (NOL) — a subsidiary of UK-based digital health startup Numan (the trading name of Vir Health Limited) — was selling food supplements for several years without registering as a Food Business Operator (FBO) — something it was required to do by law, Sifted has learned.
Under UK law, all companies that sell food items — including supplements — are required to register as an FBO 28 days before trading.
But Numan was selling supplements for at least two years and nine months before its subsidiary distribution partner NOL registered as an FBO in February this year, according to WebArchive records of its website. The supplements Numan sold during this period included vitamin D, and testosterone and fertility support.
Companies that sell food are required to register as FBOs so that their local authority can inspect premises when they need to. Some of these authorities assert that failure to register as a food business is a criminal offence.
When contacted by Sifted, Numan did not deny it had sold supplements before its subsidiary company NOL was registered as an FBO.
“Our current supplement distribution partner, who procures and delivers our supplements, is registered as a food business operator as at 14 February 2024,” said CEO and founder Sokratis Papafloratos in a statement. He added that the company received “external advice” on “applicable regulations”.
“We continually work with regulatory experts to ensure we are compliant, and continue to stay so,” he said.
The news follows Sifted’s reporting that Numan had recalled supplements that contained restricted ingredients earlier this year.
Operating
Numan has been listing supplements for sale on its website since April 2020, according to web archives, when it advertised a “fertility support” supplement that it said was to be taken daily and targeted “key areas” of male fertility.
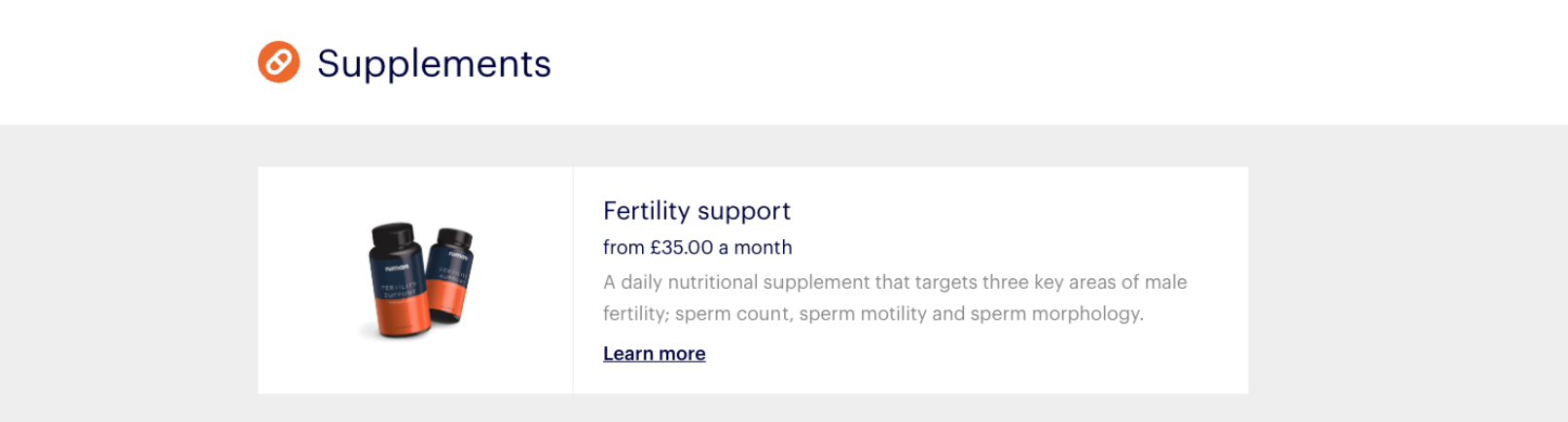
Numan had broadened that range to include supplements that it said would help improve sleep, boost testosterone and vitamin D and support prostate function by August 2021.
This was before NOL was registered as an FBO on February 14 2024.
The Food Standards Agency website, the UK’s food regulatory body, says that “to sell food supplements you must register as a Food Business Operator (FBO) with your local authority.”
Alongside supplements, Numan also prescribes medication for conditions such as erectile dysfunction, hair loss and weight loss — including weight loss jabs like Wegovy.
Backed by VNV Global and White Star Capital, the company is one of the best-funded of several European startups that have emerged in recent years selling supplements and medication online.
Since launching in 2018, Numan has raised $72.2m, according to Dealroom, most recently in a £40m ($50m) debt and equity round in February 2022. It says it has served over 500k customers.
“Operational transparency and patient safety are top priorities at Numan,” said Papafloratos in his statement to Sifted. “We make every effort to deliver this at all times, and across all areas of our organisation, as a CQC registered (and regulated) healthcare provider.”
Not the first incident at Numan
In March this year, Sifted learnt that Numan had sent out emails to customers recalling vitamin D supplements that contained Propylparaben and Methylparaben — which are restricted for use in food (including supplements) in the EU and UK.
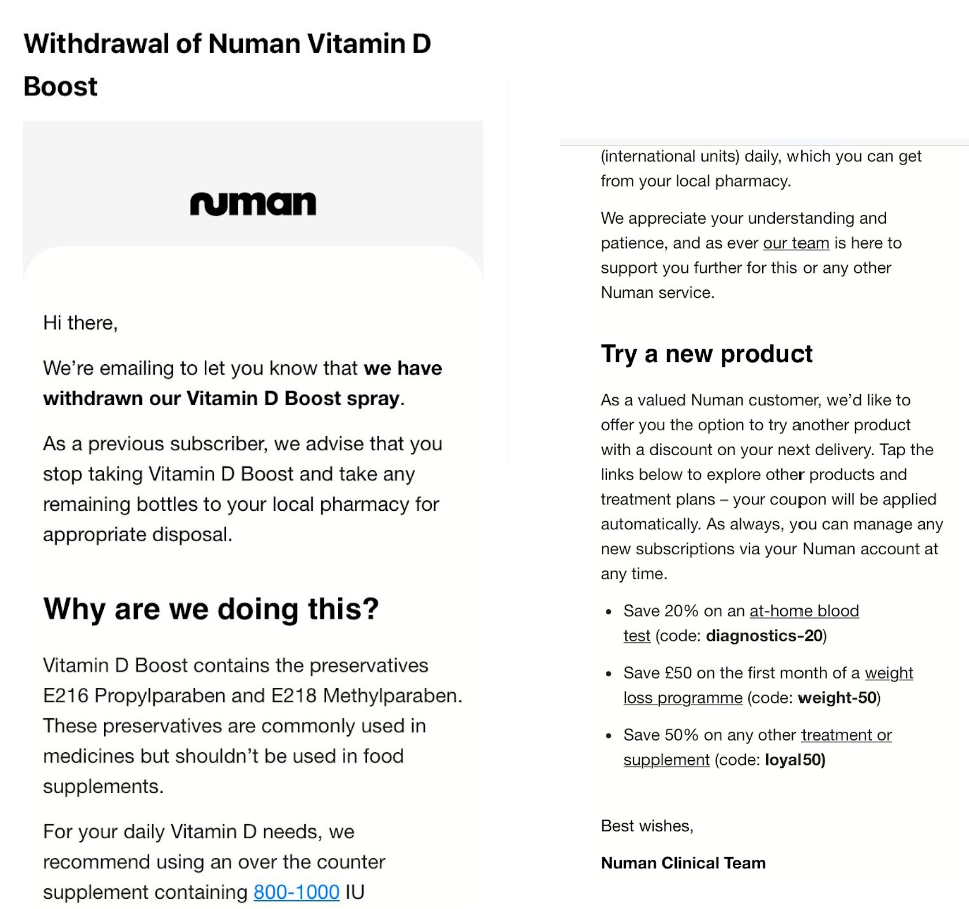
According to EU law documents, propylparaben has been shown to have effects on sex hormones and the male reproductive organs in juvenile rats.
“During an independent audit led by Numan to review the ingredients and labelling of all Numan products, we discovered that these ingredients are restricted for use in food products such as supplements,” Papafloratos told Sifted at the time. “The affected product, a Vitamin D spray supplement, that was purchased from a third party supplier, was immediately removed from sale.”
The Numan founder added at the time that the use of restricted ingredients in its vitamin D supplements was caused by an “error in the supply chain by a manufacturing partner”.
“The ingredients in question, E216 and E218 [Propylparaben and Methylparaben], are preservatives commonly used and approved for use in liquid medicinal products,” said Papafloratos. “They are safely consumed by millions of adults and children every year, in medicines like Calpol, Gaviscon and many others.
Regulator concerns
These incidents are the latest that have seen online pharmacies and digital health companies come under scrutiny in recent times.
UK healthtech Manual, which also prescribes medication online, was revealed to be selling weight loss drug Ozempic beyond a UK government deadline to stop doing so, following shortages of the drug.
There are also growing patient safety concerns linked to the rise of online vendors of supplements and medicines.
Earlier this year, a BBC investigation found 20 online pharmacies selling restricted drugs without checks like GP approval.
Duncan Rudkin, CEO of the UK pharmacy regulator the General Pharmaceutical Council, said in a statement that the investigation “raises very serious concerns”.
This article was updated on May 20 to reflect the fact that it was Numan’s distribution partner — Numan Operations Limited — that was required to register as a Food Business Operator, rather than Numan’s separate legal entity, Vir Health Limited.



